What Is The Electron Configuration Of Silicon
catholicpriest
Nov 26, 2025 · 12 min read
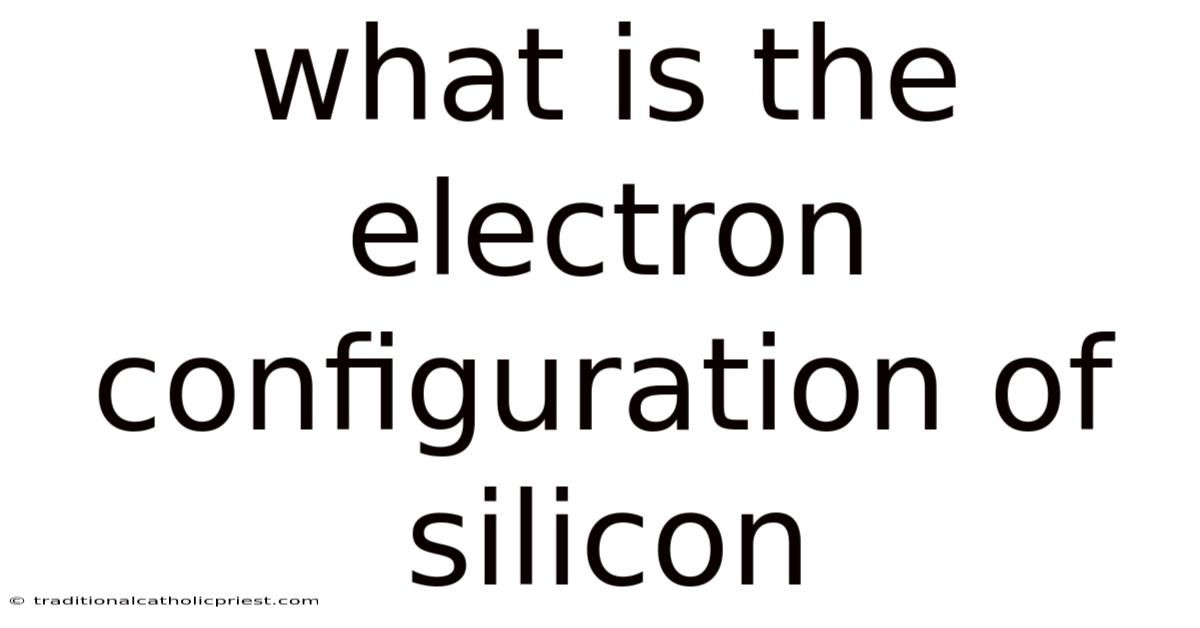
Table of Contents
Imagine peering into the very heart of matter, witnessing the intricate dance of electrons around an atom's nucleus. Each element possesses a unique choreography, a specific arrangement of these subatomic particles that dictates its behavior and properties. This is where electron configuration comes into play, acting as a fundamental roadmap for understanding the elements that make up our world.
Think of silicon, the ubiquitous element that powers our digital age. It's the backbone of computer chips, solar panels, and countless other technologies. But what gives silicon these unique properties? The answer lies within its electron configuration, a precise ordering of its electrons that governs how it interacts with other atoms. Understanding this configuration is key to unlocking silicon's secrets and harnessing its potential even further.
Understanding the Electron Configuration of Silicon
The electron configuration of an element describes the arrangement of electrons within its atoms. These electrons occupy specific energy levels and orbitals around the nucleus, following a set of rules defined by quantum mechanics. For silicon, a fascinating element crucial to modern technology, understanding its electron configuration unlocks a deeper understanding of its chemical behavior and physical properties.
Silicon (Si) has an atomic number of 14, meaning a neutral silicon atom has 14 protons in its nucleus and 14 electrons surrounding the nucleus. These electrons are not arranged randomly; instead, they fill specific energy levels and orbitals according to the Aufbau principle, Hund's rule, and the Pauli exclusion principle. The electron configuration notation provides a concise way to represent this arrangement, indicating the number of electrons in each subshell.
Comprehensive Overview of Electron Configuration
Electron configuration is the arrangement of electrons in the different energy levels and sublevels within an atom. It's governed by several fundamental principles rooted in quantum mechanics, providing a framework for understanding how electrons fill the available energy states.
Key Principles of Electron Configuration
- Aufbau Principle: This principle states that electrons first fill the lowest energy levels available before occupying higher energy levels. The order of filling generally follows the sequence: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, and 7p. This order isn't strictly followed for all elements, especially in transition metals where the energies of d and s orbitals can be very close.
- Pauli Exclusion Principle: This principle dictates that no two electrons in an atom can have the same set of four quantum numbers. This means that each orbital can hold a maximum of two electrons, and these electrons must have opposite spins (spin-up and spin-down).
- Hund's Rule: When filling degenerate orbitals (orbitals with the same energy), electrons will individually occupy each orbital before any orbital is doubly occupied. Additionally, electrons in singly occupied orbitals will have the same spin in order to minimize repulsion and achieve a more stable state.
Electron Configuration Notation
The electron configuration is typically written in a specific notation. Each subshell is represented by its principal quantum number (n) and its orbital type (s, p, d, or f), followed by a superscript indicating the number of electrons in that subshell. For example, the notation 1s² indicates that there are two electrons in the 1s subshell.
Shorthand Notation
A shorthand notation is often used to simplify the electron configuration, especially for larger atoms. This notation utilizes the noble gas that precedes the element in the periodic table. For example, sodium (Na) has the electron configuration 1s²2s²2p⁶3s¹. Neon (Ne) has the electron configuration 1s²2s²2p⁶. Therefore, the shorthand notation for sodium would be [Ne]3s¹.
Orbitals and Quantum Numbers
- Principal Quantum Number (n): This number represents the energy level of an electron and can be any positive integer (n = 1, 2, 3, ...). Higher values of n indicate higher energy levels and greater average distance from the nucleus.
- Azimuthal Quantum Number (l): This number describes the shape of the electron's orbital and ranges from 0 to n - 1. l = 0 corresponds to an s orbital (spherical shape), l = 1 corresponds to a p orbital (dumbbell shape), l = 2 corresponds to a d orbital (more complex shapes), and l = 3 corresponds to an f orbital (even more complex shapes).
- Magnetic Quantum Number (ml): This number describes the orientation of the orbital in space and can take integer values from -l to +l, including 0. For example, a p orbital (l = 1) has three possible orientations (ml = -1, 0, +1), corresponding to the px, py, and pz orbitals.
- Spin Quantum Number (ms): This number describes the intrinsic angular momentum of an electron, which is quantized and referred to as spin. Electrons behave as if they are spinning, creating a magnetic dipole moment. The spin quantum number can be either +1/2 (spin-up) or -1/2 (spin-down).
Determining the Electron Configuration of Silicon
Silicon (Si), with an atomic number of 14, has 14 electrons. We fill the orbitals according to the Aufbau principle:
- 1s subshell can hold up to 2 electrons: 1s²
- 2s subshell can hold up to 2 electrons: 1s²2s²
- 2p subshell can hold up to 6 electrons: 1s²2s²2p⁶
- 3s subshell can hold up to 2 electrons: 1s²2s²2p⁶3s²
- 3p subshell can hold up to 2 electrons: 1s²2s²2p⁶3s²3p²
Therefore, the complete electron configuration of silicon is 1s²2s²2p⁶3s²3p². The shorthand notation uses neon (Ne), which has the configuration 1s²2s²2p⁶. So, the shorthand notation for silicon is [Ne]3s²3p².
Importance of Electron Configuration
The electron configuration of an element is critical for understanding its chemical properties. The outermost electrons, also known as valence electrons, are primarily involved in chemical bonding. Silicon has four valence electrons (3s²3p²), making it capable of forming four covalent bonds. This characteristic is crucial to its ability to form stable compounds and is fundamental to its role in semiconductor technology.
The electron configuration also influences an element's ionization energy, electron affinity, and electronegativity, all of which are crucial in predicting how the element will interact with other elements in chemical reactions. By knowing the electron configuration, chemists and materials scientists can design and synthesize new materials with specific properties, tailoring them for a wide range of applications.
Trends and Latest Developments
The study of electron configurations isn't static; it's a dynamic field continuously evolving with new research and technological advancements. Current trends involve exploring the electron configurations of highly complex systems and the use of computational methods to predict and understand these configurations.
Computational Chemistry
Computational chemistry plays an increasingly vital role in determining electron configurations, particularly for complex molecules and materials. Sophisticated software packages utilize quantum mechanical calculations to approximate the electronic structure of atoms and molecules. These calculations can predict properties like bond lengths, bond angles, and vibrational frequencies, providing valuable insights into the behavior of chemical systems. Density Functional Theory (DFT) is a popular method used to calculate the electronic structure of materials, and its continued refinement improves the accuracy and applicability of these simulations.
Experimental Techniques
Experimental techniques like X-ray photoelectron spectroscopy (XPS) are used to probe the electronic structure of materials directly. XPS involves irradiating a material with X-rays and measuring the kinetic energy of the emitted electrons. By analyzing the energy spectrum of these electrons, researchers can determine the elemental composition and electronic states of the atoms within the material. This technique is particularly useful for studying surface chemistry and interfaces, providing valuable information about the oxidation states and chemical bonding of elements like silicon.
Applications in Materials Science
Understanding the electron configuration of silicon and other elements is essential for designing new materials with tailored properties. In semiconductor research, manipulating the electron configuration through doping (adding impurities) is crucial for controlling the electrical conductivity of silicon. For example, doping silicon with phosphorus (which has one more valence electron) creates an n-type semiconductor, while doping with boron (which has one fewer valence electron) creates a p-type semiconductor. The junction between n-type and p-type silicon forms the basis of transistors and other semiconductor devices.
Exotic States of Matter
Research into exotic states of matter, such as topological insulators and superconductors, often involves manipulating the electron configuration of materials at the nanoscale. Topological insulators are materials that are insulating in the bulk but have conducting surface states due to their unique electronic structure. Understanding and controlling the electron configuration is essential for realizing these novel materials with potential applications in quantum computing and spintronics.
Education and Outreach
The importance of understanding electron configurations extends to education and outreach. Chemistry educators are continuously seeking innovative ways to teach this fundamental concept effectively. Visual aids, interactive simulations, and real-world examples help students grasp the abstract concepts of quantum mechanics and electron configurations. By fostering a deeper understanding of these principles, we can inspire the next generation of scientists and engineers to develop innovative solutions to global challenges.
Tips and Expert Advice
Mastering electron configurations requires a blend of theoretical knowledge and practical application. Here are some tips and expert advice to help you understand and apply these concepts effectively:
1. Master the Basics
Before diving into complex electron configurations, make sure you have a solid grasp of the fundamental principles. Review the Aufbau principle, Hund's rule, and the Pauli exclusion principle. Understand the concept of orbitals and quantum numbers. Practice writing electron configurations for simple elements like hydrogen, helium, and oxygen. Once you have a strong foundation, you can gradually move on to more complex elements and ions.
2. Use the Periodic Table as a Guide
The periodic table is an invaluable tool for predicting electron configurations. The rows (periods) correspond to the principal quantum number (n), and the columns (groups) indicate the number of valence electrons. For example, elements in Group 1 (alkali metals) have one valence electron, while elements in Group 16 (chalcogens) have six valence electrons. By understanding the organization of the periodic table, you can quickly estimate the electron configuration of many elements.
3. Practice with Examples
The best way to learn electron configurations is to practice with numerous examples. Start with simple elements and gradually work your way up to more complex elements and ions. Write the electron configurations for elements in different groups and periods. Pay attention to exceptions to the Aufbau principle, such as chromium and copper, which have slightly different electron configurations due to the stability of half-filled and fully-filled d orbitals.
4. Visualize Orbitals
Visualizing orbitals can help you understand the spatial arrangement of electrons within an atom. Use diagrams or online simulations to visualize the shapes of s, p, and d orbitals. Understand how these orbitals are oriented in space and how they fill with electrons according to Hund's rule.
5. Understand Exceptions to the Rules
While the Aufbau principle provides a general guideline for filling orbitals, there are exceptions. For example, chromium (Cr) has the electron configuration [Ar]3d⁵4s¹, not [Ar]3d⁴4s². This is because a half-filled d subshell (d⁵) is more stable than a partially filled d subshell (d⁴). Similarly, copper (Cu) has the electron configuration [Ar]3d¹⁰4s¹, not [Ar]3d⁹4s². This is because a fully filled d subshell (d¹⁰) is more stable than a partially filled d subshell (d⁹). Understanding these exceptions can help you predict the electron configurations of elements that deviate from the Aufbau principle.
6. Use Online Resources
There are many online resources available to help you learn and practice electron configurations. Websites like Khan Academy, Chemistry LibreTexts, and ChemTube3D provide tutorials, practice problems, and interactive simulations. Use these resources to supplement your textbook and classroom learning.
7. Relate Electron Configurations to Chemical Properties
Electron configurations are not just abstract concepts; they directly influence the chemical properties of elements. For example, elements with similar valence electron configurations tend to exhibit similar chemical behavior. Alkali metals (Group 1) are highly reactive because they have one valence electron that is easily lost to form a positive ion. Halogens (Group 17) are also highly reactive because they have seven valence electrons and readily gain one electron to form a negative ion. By understanding the relationship between electron configurations and chemical properties, you can predict how elements will interact with each other in chemical reactions.
FAQ
Q: What is the electron configuration of silicon in its ground state? A: The ground state electron configuration of silicon (Si) is 1s²2s²2p⁶3s²3p².
Q: What is the shorthand notation for the electron configuration of silicon? A: The shorthand notation for silicon is [Ne]3s²3p².
Q: How many valence electrons does silicon have? A: Silicon has four valence electrons, located in the 3s and 3p subshells.
Q: Why is the electron configuration important? A: The electron configuration determines an element's chemical properties, including its ability to form bonds and its reactivity.
Q: What are the rules for determining electron configurations? A: The rules include the Aufbau principle, Hund's rule, and the Pauli exclusion principle.
Q: What is the role of electron configuration in semiconductor technology? A: Understanding the electron configuration of silicon allows us to control its electrical conductivity through doping, which is fundamental to semiconductor devices.
Conclusion
The electron configuration of silicon is a cornerstone for understanding its properties and applications. With an electron configuration of 1s²2s²2p⁶3s²3p² or [Ne]3s²3p², silicon's four valence electrons dictate its ability to form covalent bonds, making it an ideal semiconductor material. This fundamental understanding allows us to manipulate silicon's properties for use in countless electronic devices, from computer chips to solar panels.
By understanding the principles of electron configuration, you can unlock a deeper appreciation for the elements that make up our world and the technologies that shape our future. Want to delve deeper? Explore interactive periodic tables, try simulating electron configurations, or even research the latest advancements in semiconductor materials. Share your discoveries and questions in the comments below and let's continue this journey of scientific exploration together!
Latest Posts
Latest Posts
-
How To Find The Emf Of A Battery
Nov 26, 2025
-
What Are The 3 Properties Of Bases
Nov 26, 2025
-
Changes In Dna Sequence That Affect Genetic Information
Nov 26, 2025
-
What Is The Electron Configuration Of Silicon
Nov 26, 2025
-
Which Metal Is Best Conductor Of Electricity
Nov 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Silicon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.