The Periodic Table Is Based On An Element's
catholicpriest
Nov 21, 2025 · 9 min read
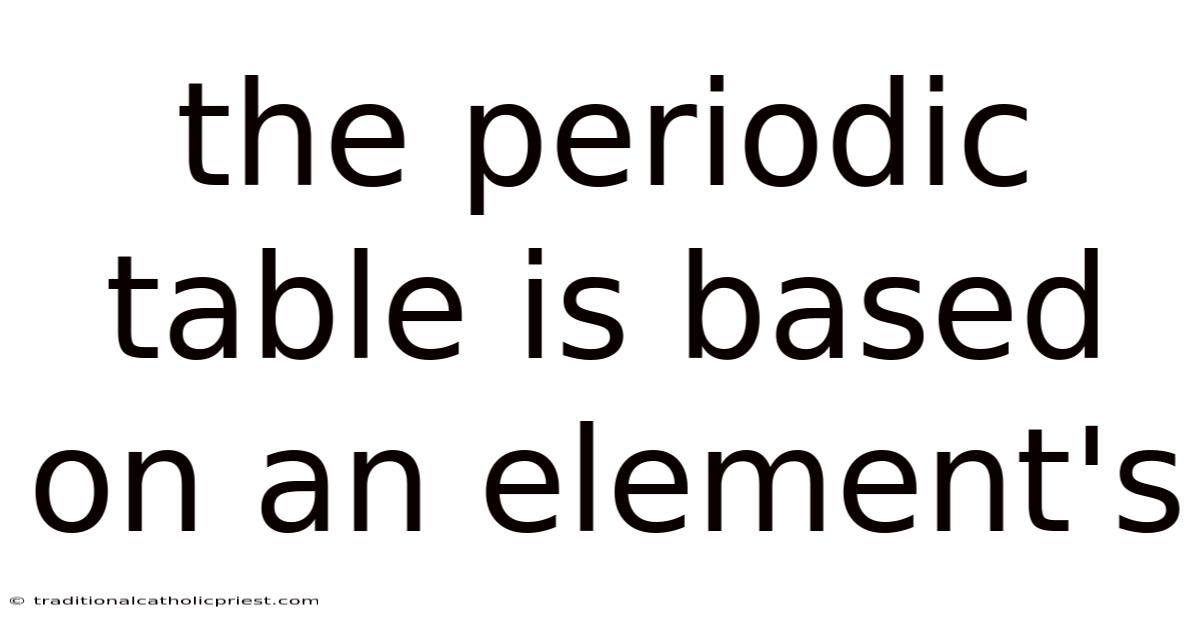
Table of Contents
Imagine trying to organize all the books in a vast library without any system. Chaos, right? That's what chemistry was like before the periodic table. Scientists knew about different elements, but understanding their relationships was a mess. Then, along came a brilliant idea: to arrange these elements based on their fundamental properties, revealing a hidden order and predictability.
Think of the periodic table as the ultimate cheat sheet for understanding how the universe is built. It's not just a list; it's a map that shows how elements relate to each other, how they'll behave, and what kind of compounds they'll form. But what's the secret code that unlocks this map? What single attribute serves as the foundation upon which this entire organization rests? The answer lies within the very core of each atom: its atomic number, dictating the number of protons.
The Foundation: Atomic Number and the Periodic Table
The periodic table is primarily organized based on an element's atomic number, which is the number of protons found in the nucleus of an atom of that element. This seemingly simple number is the key to understanding an element's chemical properties and its place in the periodic table. The arrangement starts with hydrogen, possessing a single proton (atomic number 1), and continues sequentially, each element gaining one proton as you move across and down the table.
Before the pivotal work of Henry Moseley, elements were often organized by atomic weight. However, this led to inconsistencies and anomalies. For example, iodine (atomic weight approximately 126.9) would have been placed before tellurium (atomic weight approximately 127.6), which didn't align with their observed chemical properties. Moseley's discovery that the atomic number, not the atomic weight, was the fundamental organizing principle resolved these discrepancies and solidified the modern structure of the periodic table. His work, using X-ray spectroscopy, demonstrated a clear, linear relationship between the wavelength of X-rays emitted by an element and its atomic number, confirming that the number of protons was the defining characteristic.
Comprehensive Overview: Unpacking the Significance
Defining the Element
The atomic number uniquely defines an element. Change the number of protons in an atom, and you change the element itself. For example, carbon, with six protons, is fundamentally different from nitrogen, which has seven. This is unlike neutrons, which can vary in number, creating isotopes of the same element, or electrons, which can be gained or lost to form ions. The number of protons remains constant and is the element's unchanging identifier.
Electron Configuration and Chemical Properties
The atomic number dictates the number of electrons in a neutral atom. These electrons arrange themselves in specific energy levels or shells around the nucleus. The arrangement of electrons, or the electron configuration, is what determines an element's chemical properties – how it will interact with other elements. Elements in the same group (vertical column) of the periodic table have similar electron configurations in their outermost shell (valence shell), leading to similar chemical behaviors.
Periodic Trends
The periodic table reveals trends in various properties, all stemming from the underlying organization by atomic number. Some of the most important trends include:
- Atomic Radius: Generally decreases from left to right across a period (row) due to increasing nuclear charge attracting electrons more strongly and generally increases down a group as electrons are added to higher energy levels.
- Ionization Energy: The energy required to remove an electron from an atom in the gaseous phase. It generally increases across a period due to increasing nuclear charge and decreases down a group as the outermost electrons are further from the nucleus.
- Electronegativity: The ability of an atom to attract electrons in a chemical bond. It generally increases across a period and decreases down a group, following a similar trend to ionization energy.
- Metallic Character: Generally decreases across a period as elements become less likely to lose electrons and increases down a group as elements become more likely to lose electrons.
Blocks of the Periodic Table
The periodic table can also be divided into blocks (s, p, d, and f) based on which subshell is being filled with electrons. The s-block contains groups 1 and 2 (alkali and alkaline earth metals), the p-block contains groups 13-18, the d-block contains the transition metals, and the f-block contains the lanthanides and actinides. These blocks reflect the different types of orbitals electrons occupy and contribute to the diversity of chemical properties observed across the periodic table.
Historical Context
As previously noted, before Moseley's work, the organization of elements was primarily based on atomic mass. While this worked for many elements, it led to inconsistencies. Moseley's discovery that the atomic number was the fundamental organizing principle was a paradigm shift. It not only resolved the existing inconsistencies but also allowed scientists to predict the existence and properties of undiscovered elements, filling the gaps in the periodic table. His work provided a deeper understanding of the atom's structure and the relationship between atomic structure and chemical properties.
Trends and Latest Developments
Today, the periodic table continues to evolve, though the core principle of organization by atomic number remains unchanged. While the first 118 elements have been discovered and synthesized, research continues in several exciting areas:
- Synthesis of Superheavy Elements: Scientists are pushing the boundaries of the periodic table by attempting to synthesize elements with even higher atomic numbers. These superheavy elements are extremely unstable and exist for only fractions of a second, but their study provides valuable insights into nuclear physics and the limits of nuclear stability.
- Exploring New Properties: With advanced computational methods and experimental techniques, scientists are constantly discovering new properties and behaviors of known elements, especially under extreme conditions of temperature and pressure. This is particularly relevant in fields like materials science and astrophysics.
- Isotope Research: While the atomic number defines the element, the different isotopes of an element can have significantly different properties. Research into isotopes is crucial in fields like nuclear medicine, dating techniques, and environmental science.
- Data-Driven Insights: The vast amount of data associated with the periodic table is being leveraged through data science and machine learning to identify patterns, predict new compounds, and accelerate materials discovery.
Professional insights suggest that the future of periodic table research will focus on integrating computational methods with experimental techniques to explore the properties of elements and compounds in unprecedented detail. This will lead to the development of new materials with tailored properties for a wide range of applications.
Tips and Expert Advice
Understanding the periodic table isn't just for chemists; it's a valuable tool for anyone interested in science and the world around them. Here are some practical tips and expert advice for mastering its intricacies:
-
Focus on the Fundamentals: Start by understanding the basic organization of the periodic table – periods (rows) and groups (columns). Learn the names and symbols of the common elements, such as hydrogen, oxygen, carbon, nitrogen, sodium, and chlorine. Knowing the basics will make it easier to grasp more complex concepts later on.
-
Master the Trends: Learn the major periodic trends: atomic radius, ionization energy, electronegativity, and metallic character. Understand how these trends relate to the electron configuration and nuclear charge of the elements. Practice applying these trends to predict the properties of unknown elements or compounds.
-
Understand Electron Configuration: Electron configuration is the key to understanding an element's chemical behavior. Learn how to write electron configurations using the Aufbau principle and Hund's rule. Practice relating electron configurations to the position of an element in the periodic table.
-
Use Mnemonics and Visual Aids: Create mnemonics to remember the groups of the periodic table, such as "Alkali Metals Are Not Kindly Rude Cats From France" for the alkali metals (Li, Na, K, Rb, Cs, Fr). Use visual aids, such as colored periodic tables that highlight different properties, to reinforce your understanding.
-
Relate to Real-World Examples: Connect the elements and their properties to real-world examples. For example, understand why sodium chloride (table salt) is an essential nutrient, why helium is used in balloons, or why silicon is used in computer chips. This will make the periodic table more relevant and engaging.
-
Practice, Practice, Practice: The best way to master the periodic table is to practice using it. Work through problems that require you to predict the properties of elements, write chemical formulas, or balance chemical equations. The more you practice, the more comfortable you will become with the periodic table.
-
Utilize Online Resources: There are numerous online resources available to help you learn about the periodic table, including interactive periodic tables, tutorials, and practice quizzes. Take advantage of these resources to supplement your learning.
FAQ
Q: Why is the periodic table so important?
A: The periodic table is crucial because it organizes all known elements based on their atomic structure and properties. It allows scientists to predict how elements will behave and interact, which is essential for understanding chemistry, materials science, and many other fields.
Q: What does the group number tell you?
A: The group number (vertical column) generally indicates the number of valence electrons an element has. Valence electrons are the electrons in the outermost shell, which determine an element's chemical properties.
Q: What does the period number tell you?
A: The period number (horizontal row) indicates the highest energy level occupied by electrons in that element. For example, elements in period 3 have electrons in the third energy level.
Q: Are there any elements that don't fit neatly into the periodic table?
A: Hydrogen is sometimes considered an exception because it has properties that are similar to both alkali metals (group 1) and halogens (group 17). Also, the lanthanides and actinides are often placed separately at the bottom of the table due to their unique electronic structures.
Q: How many elements are there currently in the periodic table?
A: As of now, there are 118 confirmed elements in the periodic table, filling all the rows and completing the seventh period.
Conclusion
In summary, the periodic table is fundamentally organized by an element's atomic number, the number of protons in its nucleus. This single, defining characteristic dictates the element's identity, its electron configuration, and ultimately, its chemical properties. From predicting the behavior of elements to understanding the composition of the universe, the periodic table is an indispensable tool for scientists and anyone curious about the world around them.
Now that you've explored the foundational principle behind the periodic table, take the next step. Explore an interactive periodic table online, delve into the properties of your favorite element, or even try predicting the behavior of a simple chemical reaction. The universe of chemistry awaits your exploration!
Latest Posts
Latest Posts
-
Where Is Energy Located In Atp
Nov 21, 2025
-
What Type Of Reaction Is Elephant Toothpaste
Nov 21, 2025
-
Words That Start With R Positive
Nov 21, 2025
-
Area And Perimeter Of Composite Figures
Nov 21, 2025
-
What Is The Disadvantages Of Fossil Fuels
Nov 21, 2025
Related Post
Thank you for visiting our website which covers about The Periodic Table Is Based On An Element's . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.