What Is The Molar Mass Of Acetylsalicylic Acid
catholicpriest
Nov 09, 2025 · 14 min read
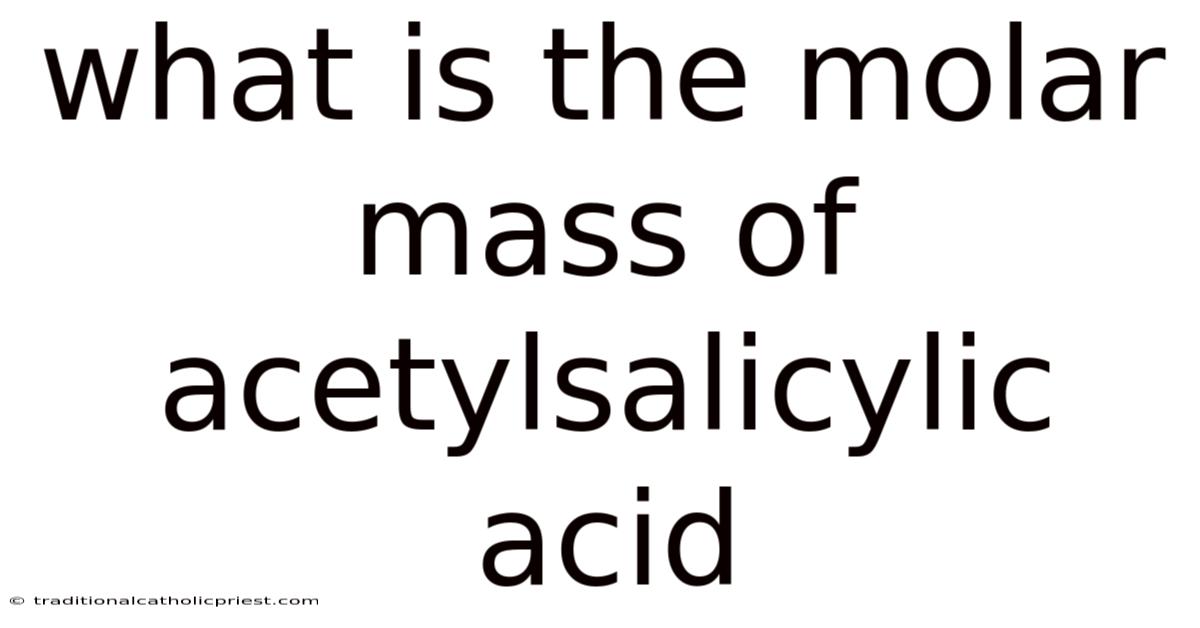
Table of Contents
Have you ever wondered what goes into that little white pill that magically alleviates your headache? Acetylsalicylic acid, more commonly known as aspirin, is a staple in medicine cabinets worldwide. From relieving minor aches to preventing heart attacks, its applications are vast and varied. But have you ever stopped to think about the chemistry behind this ubiquitous drug? Understanding its molecular structure and properties, like the molar mass of acetylsalicylic acid, is crucial for anyone interested in chemistry, pharmacology, or simply understanding the world around them.
In the realm of chemistry, determining the molar mass of acetylsalicylic acid is a fundamental exercise that bridges the gap between theoretical concepts and practical applications. It's more than just adding up atomic weights; it's about understanding the very essence of how substances interact at a molecular level. In this article, we will delve into the significance, calculation, and applications of knowing the molar mass of acetylsalicylic acid. Whether you're a student, a healthcare professional, or simply a curious mind, this comprehensive guide will illuminate the importance of this key chemical property.
Main Subheading
Acetylsalicylic acid, or aspirin, is one of the most widely used medications globally. Its fame stems from its effectiveness as an analgesic (pain reliever), antipyretic (fever reducer), and anti-inflammatory agent. But beyond its medicinal properties, acetylsalicylic acid holds a significant place in the field of chemistry. Understanding its molar mass is essential for a variety of reasons, including accurate dosing in pharmaceutical formulations, conducting stoichiometric calculations in chemical reactions, and ensuring the purity and quality of the compound in research and industrial settings.
The molar mass of a compound is the mass of one mole of that substance, expressed in grams per mole (g/mol). A mole, in turn, is a unit of measurement in chemistry that represents approximately 6.022 x 10^23 entities (atoms, molecules, ions, etc.), also known as Avogadro's number. Knowing the molar mass allows chemists and pharmacists to convert between mass and moles, which is vital for performing accurate calculations in chemical reactions and preparing solutions of specific concentrations. For acetylsalicylic acid, determining its molar mass is the cornerstone for many quantitative analyses and processes, making it a foundational concept in both theoretical and applied chemistry.
Comprehensive Overview
To truly appreciate the significance of the molar mass of acetylsalicylic acid, it is essential to understand the core definitions, scientific principles, historical context, and key concepts that underpin this chemical property. Let's delve into these elements to build a comprehensive understanding.
Definitions and Core Concepts
The molar mass of a compound is determined by summing the atomic masses of all the atoms in the molecule's chemical formula. Acetylsalicylic acid's chemical formula is C9H8O4. This means each molecule consists of 9 carbon (C) atoms, 8 hydrogen (H) atoms, and 4 oxygen (O) atoms. The atomic mass of each element can be found on the periodic table:
- Carbon (C): approximately 12.01 g/mol
- Hydrogen (H): approximately 1.008 g/mol
- Oxygen (O): approximately 16.00 g/mol
Therefore, to calculate the molar mass of acetylsalicylic acid, we use the following formula:
Molar mass = (9 × Atomic mass of C) + (8 × Atomic mass of H) + (4 × Atomic mass of O)
Molar mass = (9 × 12.01) + (8 × 1.008) + (4 × 16.00)
Molar mass = 108.09 + 8.064 + 64.00
Molar mass ≈ 180.15 g/mol
Thus, the molar mass of acetylsalicylic acid is approximately 180.15 grams per mole.
Scientific Foundations
The concept of molar mass is rooted in the atomic theory proposed by John Dalton in the early 19th century. Dalton posited that all matter is composed of atoms, which are indivisible and have distinct weights. This laid the groundwork for understanding the relative masses of different elements. Later, Amedeo Avogadro proposed that equal volumes of gases, at the same temperature and pressure, contain the same number of molecules, leading to the concept of the mole and Avogadro's number.
These scientific advancements allowed chemists to quantify the amount of substance in a way that accounts for the atomic nature of matter. The molar mass serves as a conversion factor between the mass of a substance and the number of moles, enabling precise calculations in chemical reactions. In the context of acetylsalicylic acid, knowing its molar mass allows for accurate measurement and dosing, ensuring the drug's effectiveness and safety.
Historical Context
Acetylsalicylic acid was first synthesized by Felix Hoffmann, a chemist at Bayer, in 1897. Hoffmann was searching for a less irritating alternative to salicylic acid, a natural compound derived from willow bark that had been used for centuries to treat pain and fever. By acetylating salicylic acid, Hoffmann created a compound that was more palatable and less harsh on the stomach.
Bayer patented acetylsalicylic acid under the name "Aspirin," and it quickly became a blockbuster drug. Its widespread adoption revolutionized pain management and marked the beginning of the modern pharmaceutical industry. From its early days as a simple pain reliever, acetylsalicylic acid has been extensively studied, revealing its multifaceted properties and applications. Today, it remains one of the most widely used medications in the world, a testament to its efficacy and versatility.
Significance in Chemistry and Pharmacology
In both chemistry and pharmacology, the molar mass of acetylsalicylic acid is crucial for several reasons:
-
Stoichiometry: In chemical reactions involving acetylsalicylic acid, knowing its molar mass allows chemists to calculate the exact amounts of reactants and products needed to achieve desired outcomes. This is essential for synthesizing the compound and studying its reactions with other substances.
-
Pharmaceutical Formulations: In pharmaceutical manufacturing, accurate dosing is paramount. The molar mass of acetylsalicylic acid is used to determine the precise amount of the drug needed in each tablet or capsule, ensuring that patients receive the correct dosage.
-
Analytical Chemistry: Analytical techniques such as titration, spectroscopy, and chromatography rely on the molar mass to quantify the amount of acetylsalicylic acid in a sample. This is important for quality control, research, and forensic analysis.
-
Research and Development: Researchers studying the effects of acetylsalicylic acid on biological systems need to know its molar mass to calculate concentrations, dosages, and other relevant parameters.
Importance of Accurate Determination
The accuracy of the molar mass value is critical. Even small errors in the atomic masses used in the calculation can lead to significant discrepancies in experimental results and pharmaceutical formulations. For example, if the molar mass is slightly off, the concentration of a solution prepared using that value will also be incorrect.
In pharmaceutical manufacturing, this could result in under- or over-dosing of medications, with potentially serious consequences for patients. Therefore, chemists and pharmacists rely on precise atomic mass values and careful calculations to ensure the accuracy of the molar mass of acetylsalicylic acid. Advanced techniques such as mass spectrometry can also be used to experimentally verify the molar mass and ensure the purity of the compound.
Trends and Latest Developments
In recent years, several trends and developments have influenced the understanding and application of acetylsalicylic acid's molar mass. These include advancements in analytical techniques, new research into its therapeutic uses, and evolving perspectives on personalized medicine.
Analytical Techniques
Modern analytical techniques have significantly improved the accuracy and precision of molar mass determination. Mass spectrometry, in particular, has become an indispensable tool for verifying the molar mass and purity of acetylsalicylic acid. High-resolution mass spectrometers can measure the mass-to-charge ratio of ions with extreme accuracy, allowing for the precise determination of the molecular weight. This is especially useful for identifying impurities and ensuring the quality of pharmaceutical products.
Other techniques such as nuclear magnetic resonance (NMR) spectroscopy and infrared (IR) spectroscopy also provide valuable information about the structure and composition of acetylsalicylic acid. These methods, combined with mass spectrometry, offer a comprehensive approach to characterizing the compound and confirming its molar mass.
Therapeutic Uses
While acetylsalicylic acid has been used for over a century as a pain reliever and fever reducer, ongoing research continues to uncover new therapeutic uses. Low-dose aspirin is commonly prescribed to prevent heart attacks and strokes by inhibiting platelet aggregation. However, recent studies have explored its potential in other areas, such as cancer prevention and treatment.
Researchers are investigating the mechanisms by which acetylsalicylic acid exerts its effects on various biological pathways. Understanding the molecular interactions and biochemical processes involved requires precise knowledge of the compound's molar mass. This allows scientists to calculate concentrations, dosages, and other relevant parameters in their experiments.
Personalized Medicine
The field of personalized medicine aims to tailor medical treatments to individual patients based on their genetic makeup, lifestyle, and other factors. Acetylsalicylic acid is one of the drugs being studied in this context. Researchers are exploring how genetic variations can affect a patient's response to aspirin, including its efficacy and potential side effects.
Knowing the molar mass of acetylsalicylic acid is essential for conducting these studies. It allows scientists to accurately measure the drug's concentration in patient samples and correlate it with clinical outcomes. This information can be used to develop personalized dosing strategies that maximize the benefits of acetylsalicylic acid while minimizing the risks.
Data and Popular Opinions
The scientific community widely accepts the molar mass of acetylsalicylic acid as approximately 180.15 g/mol. This value is based on the most recent atomic mass data published by the International Union of Pure and Applied Chemistry (IUPAC). However, it's important to note that the exact value may vary slightly depending on the source and the level of precision used in the calculation.
In popular opinion, acetylsalicylic acid is often viewed as a safe and effective medication. However, it's essential to be aware of the potential side effects, such as stomach irritation and bleeding. Healthcare professionals generally advise patients to consult with their doctor before taking aspirin, especially if they have a history of gastrointestinal problems or are taking other medications.
Tips and Expert Advice
Understanding the molar mass of acetylsalicylic acid is one thing; applying that knowledge effectively is another. Here are some practical tips and expert advice to help you use this information in real-world scenarios.
Accurate Calculations
When calculating the molar mass of acetylsalicylic acid, always use the most up-to-date atomic mass values from a reliable source, such as the IUPAC. Be sure to include all the atoms in the chemical formula and pay attention to the units. Double-check your calculations to avoid errors.
For complex calculations, consider using a scientific calculator or a computer program. These tools can help you perform the calculations more quickly and accurately. Also, be mindful of significant figures. The final answer should be rounded to the appropriate number of significant figures based on the least precise value used in the calculation.
Pharmaceutical Compounding
In pharmaceutical compounding, accuracy is paramount. When preparing medications containing acetylsalicylic acid, always use a calibrated balance to weigh the ingredients. Verify the molar mass of acetylsalicylic acid and use it to calculate the exact amount needed for the desired concentration.
Follow proper compounding techniques and quality control procedures to ensure the safety and efficacy of the medication. Keep detailed records of all ingredients and calculations. If you are unsure about any aspect of the compounding process, consult with a qualified pharmacist or pharmaceutical scientist.
Research Applications
In research settings, the molar mass of acetylsalicylic acid is used in a variety of applications, such as preparing solutions, conducting experiments, and analyzing data. When preparing solutions, use a volumetric flask to ensure accurate concentration. Clearly label all solutions with the compound name, concentration, and date of preparation.
When designing experiments, consider the potential effects of acetylsalicylic acid on the biological system being studied. Use appropriate controls and replicates to minimize variability and ensure the reliability of your results. When analyzing data, use statistical methods to determine the significance of your findings.
Safety Precautions
Acetylsalicylic acid can cause side effects, such as stomach irritation and bleeding. Always follow safety precautions when handling the compound. Wear appropriate personal protective equipment, such as gloves and eye protection. Avoid inhaling or ingesting the compound.
If you experience any adverse effects, such as nausea, vomiting, or abdominal pain, stop taking the medication and consult with a healthcare professional. Keep acetylsalicylic acid out of reach of children and pets. Store it in a cool, dry place away from direct sunlight.
Real-World Examples
To illustrate the practical applications of the molar mass of acetylsalicylic acid, consider the following examples:
-
Preparing an Aspirin Solution: Suppose you want to prepare a 100 mL solution of aspirin with a concentration of 1 mg/mL. To calculate the amount of aspirin needed, you would first determine the desired mass of aspirin in the solution:
Mass = Concentration × Volume
Mass = 1 mg/mL × 100 mL
Mass = 100 mg = 0.1 g
Using the molar mass of acetylsalicylic acid (180.15 g/mol), you can calculate the number of moles of aspirin needed:
Moles = Mass / Molar mass
Moles = 0.1 g / 180.15 g/mol
Moles ≈ 0.000555 mol
This calculation ensures you add the precise amount of aspirin to achieve the desired concentration.
-
Stoichiometric Calculations: In a research laboratory, if you are synthesizing acetylsalicylic acid from salicylic acid and acetic anhydride, you need to know the molar mass of acetylsalicylic acid to calculate the theoretical yield of the reaction. This allows you to determine the efficiency of the synthesis process.
-
Quality Control: In a pharmaceutical company, the molar mass of acetylsalicylic acid is used to verify the purity of the drug. Techniques like titration or HPLC (High-Performance Liquid Chromatography) are used, and the results are compared against the expected values based on the molar mass to ensure the product meets quality standards.
FAQ
Q: What is the chemical formula of acetylsalicylic acid?
A: The chemical formula of acetylsalicylic acid is C9H8O4.
Q: How is the molar mass of acetylsalicylic acid calculated?
A: The molar mass is calculated by summing the atomic masses of all the atoms in the chemical formula: (9 × Atomic mass of C) + (8 × Atomic mass of H) + (4 × Atomic mass of O).
Q: What is the molar mass of acetylsalicylic acid?
A: The molar mass of acetylsalicylic acid is approximately 180.15 g/mol.
Q: Why is it important to know the molar mass of acetylsalicylic acid?
A: Knowing the molar mass is crucial for accurate dosing in pharmaceutical formulations, conducting stoichiometric calculations in chemical reactions, and ensuring the purity and quality of the compound.
Q: Where can I find the most accurate atomic mass values?
A: The most accurate atomic mass values can be found on the IUPAC website or in reputable chemistry textbooks.
Q: What are some common uses of acetylsalicylic acid?
A: Acetylsalicylic acid is commonly used as an analgesic (pain reliever), antipyretic (fever reducer), and anti-inflammatory agent. It is also used to prevent heart attacks and strokes.
Q: Are there any safety precautions I should be aware of when handling acetylsalicylic acid?
A: Yes, acetylsalicylic acid can cause side effects, such as stomach irritation and bleeding. Always follow safety precautions, such as wearing gloves and eye protection, and consult with a healthcare professional if you experience any adverse effects.
Conclusion
In summary, the molar mass of acetylsalicylic acid, approximately 180.15 g/mol, is a fundamental concept in chemistry and pharmacology with far-reaching implications. From enabling precise stoichiometric calculations to ensuring accurate dosing in pharmaceutical formulations, its significance cannot be overstated. Understanding the scientific foundations, historical context, and latest developments related to acetylsalicylic acid's molar mass is essential for anyone working in these fields.
By applying the tips and expert advice provided in this article, you can confidently use this knowledge in real-world scenarios. Whether you are preparing an aspirin solution, conducting research experiments, or ensuring the quality of pharmaceutical products, a solid understanding of the molar mass of acetylsalicylic acid will help you achieve accurate and reliable results. Now, take the next step: explore further resources, conduct your own experiments, and deepen your understanding of this fascinating and important compound. Share this article to help others understand the importance of this crucial concept!
Latest Posts
Latest Posts
-
What Is The Difference Between A Row And Column
Nov 09, 2025
-
What Are The Parts Of Lithosphere
Nov 09, 2025
-
8 Out Of 9 As A Percentage
Nov 09, 2025
-
What Does The Root Phile Mean
Nov 09, 2025
-
How To Identify Functions On A Graph
Nov 09, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Acetylsalicylic Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.