Is Mol/l The Same As M
catholicpriest
Nov 28, 2025 · 11 min read
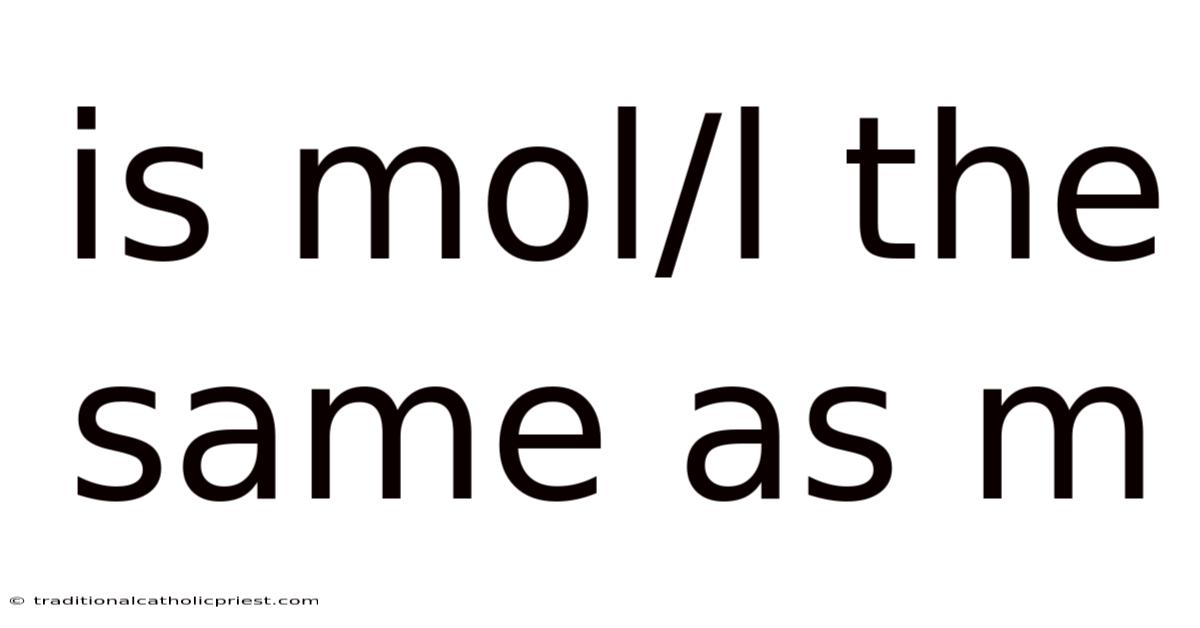
Table of Contents
Imagine you're baking a cake. You meticulously measure your ingredients – a cup of flour, a teaspoon of vanilla, a precisely measured amount of sugar. Each ingredient plays a crucial role, and getting the proportions right is essential for a delicious outcome. In chemistry, much like baking, precise measurements are paramount. We need to know how much of a substance is present in a solution to understand and control chemical reactions. This is where molarity comes in, a fundamental concept represented as mol/L, which helps us quantify the concentration of solutions.
But here's where things can get a bit confusing: you might stumble upon the abbreviation "M" used interchangeably with "mol/L." Is it simply a shorthand? Does it represent something slightly different? The answer, while seemingly straightforward, often gets muddled. Understanding the nuances between "mol/L" and "M" is crucial for accurately interpreting scientific literature, performing calculations, and ultimately, conducting successful experiments. Let’s dive deep into the world of molarity and clear up any confusion surrounding these two representations.
Main Subheading
Molarity, the measure of concentration expressed in moles per liter (mol/L), is a cornerstone of quantitative chemistry. It provides a standardized way to describe the amount of a solute dissolved in a given volume of solvent. This standardization allows chemists around the globe to communicate and replicate experiments with precision. However, the shorthand "M" is frequently used to represent molarity, which can lead to misunderstandings if not properly defined and understood. The crux of the matter lies in recognizing that while "M" is a convenient symbol, it inherently stands for "mol/L." Neglecting this underlying unit can lead to errors in calculations and misinterpretations of experimental results.
The importance of understanding molarity extends beyond the laboratory. It plays a vital role in fields as diverse as medicine, environmental science, and materials science. For example, in medicine, the concentration of a drug in a patient's bloodstream directly affects its efficacy and potential toxicity. Similarly, in environmental science, monitoring the concentration of pollutants in water sources is crucial for assessing environmental impact and implementing remediation strategies. A solid grasp of molarity, and its representation as both mol/L and M, is therefore essential for anyone working in these fields.
Comprehensive Overview
Molarity, symbolized as 'M' or expressed in its full form as 'mol/L' (moles per liter), fundamentally quantifies the concentration of a solution. It is defined as the number of moles of a solute dissolved in one liter of solution. This definition is crucial because it directly links the amount of substance (in moles) to a defined volume (in liters), providing a standardized way to express concentration.
To fully grasp molarity, understanding the underlying concepts of moles, solutes, and solutions is necessary. A mole is a unit of measurement that represents a specific number of particles (atoms, molecules, ions, etc.), specifically 6.022 x 10²³, also known as Avogadro's number. The solute is the substance being dissolved, while the solvent is the substance in which the solute dissolves. The resulting homogeneous mixture is called the solution.
The mathematical formula for molarity is straightforward:
Molarity (M) = Moles of Solute / Liters of Solution
For example, if you dissolve 1 mole of sodium chloride (NaCl) in enough water to make 1 liter of solution, the molarity of the solution is 1 mol/L, or simply 1 M. This means that every liter of this solution contains 1 mole of NaCl.
Historically, the concept of molarity arose from the need for a consistent and reproducible method for expressing concentrations in chemical reactions. Before the widespread adoption of molarity, various other methods were used, often leading to inconsistencies and difficulties in replicating experimental results. The standardization of molarity provided a common language for chemists, facilitating communication and collaboration.
Molarity differs from other measures of concentration such as molality (moles of solute per kilogram of solvent) and normality (gram equivalent weight of solute per liter of solution). While all these measures quantify concentration, they do so using different parameters. Molality, unlike molarity, is independent of temperature because it is based on mass rather than volume, which can change with temperature. Normality, on the other hand, is specific to the type of chemical reaction (e.g., acid-base or redox) and represents the number of reactive units per liter of solution. Molarity is generally preferred for most applications due to its simplicity and direct relationship to the number of moles of solute.
It is important to note that when preparing solutions of a specific molarity, the volume refers to the final volume of the solution, not just the volume of the solvent added. This distinction is crucial because the addition of the solute can slightly change the volume of the solution. To ensure accuracy, volumetric flasks are used, which are calibrated to contain a precise volume at a specific temperature. The solute is added to the flask, and then the solvent is added until the solution reaches the calibration mark on the flask.
Trends and Latest Developments
The use of molarity remains a fundamental practice in chemistry, but its application is evolving with advancements in technology and research. One notable trend is the increasing emphasis on microfluidics and nanomaterials, which require precise control and manipulation of solutions at extremely small scales. In these contexts, molarity is still used to define concentrations, but the volumes involved are often in the microliter or nanoliter range. Specialized techniques and equipment are needed to prepare and handle these highly concentrated or diluted solutions accurately.
Another significant development is the growing use of computational chemistry and simulations. These simulations often require accurate input data, including the concentrations of reactants and products. Molarity provides a standardized way to express these concentrations, ensuring the reliability and reproducibility of the simulations. Furthermore, advancements in analytical techniques, such as mass spectrometry and chromatography, allow for the precise determination of molar concentrations in complex mixtures. These techniques are crucial for monitoring reaction kinetics, identifying unknown compounds, and quantifying trace amounts of substances in various samples.
A recent trend in chemical education is the use of interactive simulations and virtual labs to teach the concept of molarity. These tools allow students to visualize the dissolution process, manipulate concentrations, and observe the effects of changing molarity on chemical reactions. This hands-on approach can enhance understanding and make learning more engaging.
Despite its widespread use, there is an ongoing discussion in the scientific community about the limitations of molarity. As mentioned earlier, molarity is temperature-dependent, which can be a significant drawback in certain applications. To address this issue, researchers are exploring alternative measures of concentration, such as molality and mole fraction, which are temperature-independent. However, molarity remains the most commonly used measure of concentration due to its simplicity and convenience. The scientific literature consistently uses both 'M' and 'mol/L' interchangeably, reinforcing the importance of understanding their equivalence.
Modern research increasingly focuses on developing more sustainable and environmentally friendly chemical processes. This often involves optimizing reaction conditions to minimize waste and maximize efficiency. Molarity plays a crucial role in these efforts by allowing researchers to precisely control the stoichiometry of reactions and identify the optimal concentrations of catalysts and other reagents.
Tips and Expert Advice
Working with molarity requires careful attention to detail to ensure accuracy and avoid errors. Here are some practical tips and expert advice to help you master this fundamental concept:
-
Always specify units: While "M" is a convenient abbreviation, it's essential to remember that it represents "mol/L." When performing calculations or reporting results, always include the units to avoid ambiguity. For instance, write "0.5 M NaCl" or "0.5 mol/L NaCl" instead of simply "0.5 NaCl." This practice makes it clear that you are referring to molarity and not some other measure of concentration.
-
Use volumetric flasks for accurate dilutions: When preparing solutions of a specific molarity, use volumetric flasks instead of graduated cylinders or beakers. Volumetric flasks are designed to contain a precise volume at a specific temperature, ensuring the accuracy of your solution. Fill the flask to the mark carefully, using a dropper to add the final few drops.
-
Account for the volume of the solute: When dissolving a solid solute in a solvent, the volume of the solute can slightly increase the total volume of the solution. To accurately prepare a solution of a specific molarity, dissolve the solute in a volume of solvent slightly less than the desired final volume. Then, add more solvent until the solution reaches the calibration mark on the volumetric flask.
-
Consider temperature effects: Molarity is temperature-dependent because the volume of a solution can change with temperature. If you need to prepare a solution at a specific temperature, make sure to bring the solvent and solute to that temperature before mixing them. For highly precise work, consider using molality instead, as it is temperature-independent.
-
Double-check your calculations: Before preparing a solution, carefully double-check your calculations to ensure that you are using the correct amount of solute and solvent. A small error in your calculations can lead to a significant error in the molarity of the solution. Use a calculator or spreadsheet to perform the calculations and verify your results.
-
Properly label your solutions: Once you have prepared a solution, label it clearly with the name of the solute, its molarity, the date of preparation, and your initials. This will help you keep track of your solutions and avoid confusion. Store solutions in appropriate containers and follow any specific storage instructions.
-
Understand dilution calculations: Dilution is the process of reducing the concentration of a solution by adding more solvent. The formula for dilution is:
M₁V₁ = M₂V₂
Where:
- M₁ is the initial molarity
- V₁ is the initial volume
- M₂ is the final molarity
- V₂ is the final volume
This formula can be used to calculate the volume of a concentrated solution needed to prepare a diluted solution of a specific molarity.
-
Practice, practice, practice: The best way to master molarity is to practice solving problems and preparing solutions. Work through examples in your textbook or online, and try preparing solutions of different molarities in the lab. The more you practice, the more comfortable you will become with the concept and the easier it will be to apply it in your work.
FAQ
Q: What does 'M' stand for in chemistry?
A: 'M' is the symbol for molarity, which represents the concentration of a solution in moles of solute per liter of solution (mol/L).
Q: Is there a difference between 'M' and 'mol/L'?
A: No, 'M' and 'mol/L' are equivalent. 'M' is simply a shorthand notation for 'mol/L'.
Q: Why is molarity important?
A: Molarity is crucial for expressing the concentration of solutions in a standardized way, enabling accurate and reproducible chemical experiments and calculations.
Q: Is molarity temperature-dependent?
A: Yes, molarity is temperature-dependent because the volume of a solution can change with temperature.
Q: What is the formula for calculating molarity?
A: Molarity (M) = Moles of Solute / Liters of Solution
Q: How do I prepare a solution of a specific molarity?
A: Dissolve the calculated amount of solute in a volume of solvent slightly less than the desired final volume. Then, add more solvent until the solution reaches the calibration mark on a volumetric flask.
Q: What is the difference between molarity and molality?
A: Molarity is moles of solute per liter of solution, while molality is moles of solute per kilogram of solvent. Molality is temperature-independent, while molarity is not.
Q: When should I use molality instead of molarity?
A: Use molality when the temperature of the solution is likely to change or when precise concentration measurements are needed at different temperatures.
Q: How do I dilute a solution of a specific molarity?
A: Use the dilution formula M₁V₁ = M₂V₂ to calculate the volume of the concentrated solution needed to prepare the diluted solution.
Conclusion
Understanding molarity, represented as both mol/L and M, is essential for anyone working in chemistry or related fields. While 'M' serves as a convenient shorthand, remembering its equivalence to 'mol/L' is crucial for accurate calculations and interpretations. By grasping the concepts, trends, and practical tips discussed in this article, you can confidently work with solutions and apply molarity in your experiments and research.
Now that you have a solid understanding of molarity, put your knowledge to the test! Try solving some practice problems, prepare solutions of different molarities in the lab, and explore the various applications of molarity in your field of study. Share your experiences and insights in the comments below, and let's continue learning and growing together in the fascinating world of chemistry!
Latest Posts
Latest Posts
-
An Element Is A Substance That
Nov 28, 2025
-
What Is Archaebacteria Cell Wall Made Of
Nov 28, 2025
-
Compare And Contrast Skeletal Cardiac And Smooth Muscle
Nov 28, 2025
-
Which Of The Following Form The Front Of The Foot
Nov 28, 2025
-
Change A Percentage To A Fraction
Nov 28, 2025
Related Post
Thank you for visiting our website which covers about Is Mol/l The Same As M . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.