If An Atom Gains An Electron It Becomes
catholicpriest
Nov 18, 2025 · 11 min read
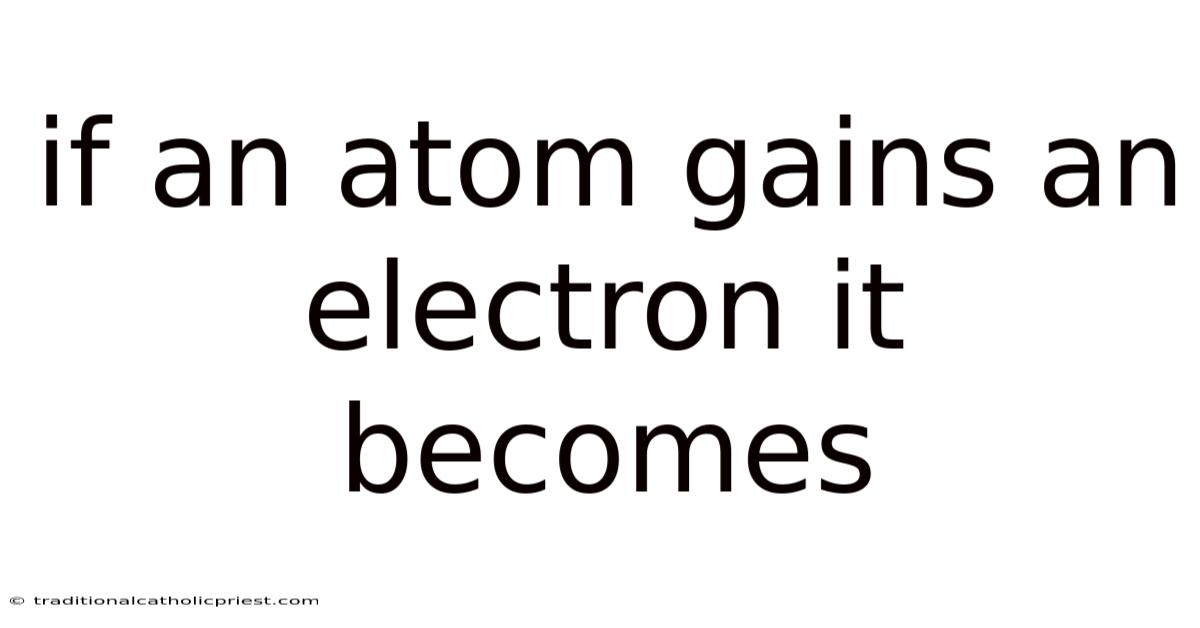
Table of Contents
Imagine walking through a bustling marketplace, where atoms are like tiny shoppers, each looking to complete their purchase. Some atoms are eager to grab an extra item off the shelf—in this case, an electron—to feel more content and stable. It's all about achieving a balanced state, a bit like finding the perfect ingredients for a recipe.
Now, picture a specific atom, perhaps chlorine (Cl), floating around with a slight sense of incompleteness. Chlorine, with its 17 electrons, is just one electron shy of having a full outer shell, the magic number that brings stability. When chlorine bumps into an electron, maybe one that’s been detached from a sodium (Na) atom, it grabs it eagerly. In that simple act, chlorine transforms, becoming something new—a negatively charged ion.
Understanding What Happens When an Atom Gains an Electron
Atoms, the fundamental building blocks of matter, are electrically neutral in their natural state. This neutrality arises from having an equal number of positively charged protons in their nucleus and negatively charged electrons orbiting around the nucleus. However, atoms don't always stay neutral. They can gain or lose electrons to achieve a more stable electron configuration, similar to how people might seek balance in their lives. When an atom gains an electron, it disrupts this electrical balance, leading to the formation of an ion. Specifically, when an atom gains one or more electrons, it becomes a negatively charged ion, known as an anion.
The process of gaining an electron is fundamental to understanding chemical reactions and the formation of chemical compounds. It’s a concept that bridges basic atomic structure with the behavior of matter at a macroscopic scale. This article will delve into the details of what happens when an atom gains an electron, exploring the underlying principles, real-world examples, and implications of this process.
Comprehensive Overview: The Science Behind Electron Gain
To fully grasp what occurs when an atom gains an electron, it’s essential to understand some core concepts of atomic structure and chemical behavior.
Atomic Structure Revisited
Atoms consist of three primary particles: protons, neutrons, and electrons. Protons, located in the nucleus, carry a positive charge. Neutrons, also in the nucleus, have no charge. Electrons, which are negatively charged, orbit the nucleus in specific energy levels or shells. The number of protons defines the element's atomic number and determines its identity.
The arrangement of electrons in these shells is governed by quantum mechanics. The innermost shell can hold up to two electrons, while the second and third shells can hold up to eight electrons each. Atoms are most stable when their outermost electron shell, or valence shell, is full. This is known as the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a full valence shell of eight electrons.
Ionization: The Formation of Ions
Ionization is the process by which an atom gains or loses electrons, resulting in the formation of an ion. When an atom gains electrons, it becomes a negatively charged ion, or anion. Conversely, when an atom loses electrons, it becomes a positively charged ion, or cation. The charge of an ion is determined by the difference between the number of protons and electrons.
For example, consider a neutral chlorine (Cl) atom, which has 17 protons and 17 electrons. If chlorine gains one electron, it will then have 17 protons and 18 electrons, resulting in a net charge of -1. This negatively charged chlorine ion is written as Cl⁻. Similarly, if a sodium (Na) atom, which has 11 protons and 11 electrons, loses one electron, it will have 11 protons and 10 electrons, resulting in a net charge of +1. This positively charged sodium ion is written as Na⁺.
Electronegativity and Electron Affinity
Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. Atoms with high electronegativity values have a strong pull on electrons, while those with low electronegativity values have a weaker pull. This property plays a crucial role in determining whether an atom is likely to gain or lose electrons.
Electron affinity is the energy change that occurs when an electron is added to a neutral atom in the gaseous phase. If energy is released when an electron is added (i.e., the energy change is negative), the atom has a high electron affinity and readily accepts electrons. Conversely, if energy is required to add an electron (i.e., the energy change is positive), the atom has a low electron affinity and does not easily accept electrons.
Elements such as halogens (e.g., fluorine, chlorine, bromine) have high electronegativity and high electron affinity, making them prone to gaining electrons and forming anions. On the other hand, alkali metals (e.g., lithium, sodium, potassium) have low electronegativity and low electron affinity, making them more likely to lose electrons and form cations.
The Driving Force Behind Electron Gain
The primary driving force behind an atom gaining an electron is to achieve a stable electron configuration. Atoms strive to have a full valence shell, which typically means having eight electrons (except for elements like hydrogen and helium, which aim for two electrons). By gaining electrons, atoms can attain this stability, mimicking the electron configuration of noble gases, which are exceptionally stable and unreactive.
For instance, oxygen (O), with six valence electrons, needs two more electrons to complete its octet. It readily gains two electrons to form an oxide ion (O²⁻), which has the same electron configuration as neon (Ne), a noble gas. This drive for stability explains why certain elements are highly reactive and readily form ions.
Trends and Latest Developments in Understanding Anion Formation
In recent years, advancements in computational chemistry and experimental techniques have deepened our understanding of anion formation. Researchers are exploring the dynamics of electron attachment to atoms and molecules with unprecedented precision.
Computational Chemistry Insights
Computational chemistry, employing sophisticated algorithms and high-performance computing, allows scientists to model and predict the behavior of atoms and molecules. These simulations can accurately calculate electron affinities, ionization energies, and electronegativity values, providing insights into the likelihood of an atom gaining or losing electrons.
Density Functional Theory (DFT) is one of the most widely used computational methods for studying electron behavior in atoms and molecules. DFT calculations can predict the electronic structure of anions, revealing how the extra electron is distributed around the atom and how it affects the overall stability of the ion.
Experimental Techniques
Experimental techniques, such as photoelectron spectroscopy and Rydberg electron transfer, provide direct measurements of electron affinities and ionization energies. Photoelectron spectroscopy involves bombarding atoms or molecules with photons and measuring the kinetic energy of the ejected electrons. This technique can reveal the energy levels of electrons in anions, providing valuable information about their electronic structure.
Rydberg electron transfer involves transferring an electron from a Rydberg atom (an atom with an electron in a very high energy level) to a neutral atom or molecule. This technique allows for the precise measurement of electron affinities and can be used to study the formation of highly unstable anions.
Current Trends
One significant trend in the field is the exploration of superhalogens. Superhalogens are clusters of atoms that exhibit even higher electron affinities than individual halogen atoms. For example, the AlCl₄ cluster has a higher electron affinity than chlorine, making it an exceptionally strong oxidizing agent. Research into superhalogens could lead to the development of novel materials and chemical reactions.
Another emerging area is the study of multiply charged anions. While most stable anions have a charge of -1 or -2, researchers are exploring the possibility of creating and stabilizing anions with higher negative charges. These multiply charged anions could have unique properties and applications in areas such as energy storage and catalysis.
Tips and Expert Advice on Understanding Electron Gain
Understanding how atoms gain electrons can be challenging. Here are some tips and expert advice to help clarify the concepts:
Visualize Electron Configurations
One of the best ways to understand electron gain is to visualize electron configurations. Draw diagrams of atoms showing the number of protons in the nucleus and the arrangement of electrons in different energy levels or shells. This visual representation can make it easier to see how atoms gain electrons to achieve a full valence shell.
For example, draw the electron configuration of a chlorine atom (17 protons and 17 electrons) with two electrons in the first shell, eight electrons in the second shell, and seven electrons in the third shell. Then, draw the electron configuration of a chloride ion (Cl⁻) with 18 electrons, showing a complete octet in the third shell.
Use the Periodic Table as a Guide
The periodic table is an invaluable tool for predicting whether an atom is likely to gain or lose electrons. Elements in the same group (vertical column) have similar valence electron configurations and tend to exhibit similar chemical behavior.
For example, elements in Group 17 (halogens) all have seven valence electrons and readily gain one electron to form anions with a -1 charge. Elements in Group 16 (chalcogens) have six valence electrons and tend to gain two electrons to form anions with a -2 charge. Understanding these trends can help you predict the ionic charges of different elements.
Practice with Real-World Examples
Practice applying the concepts of electron gain to real-world examples. Consider the formation of common ionic compounds such as sodium chloride (NaCl) and magnesium oxide (MgO).
In sodium chloride, sodium (Na) loses one electron to form a Na⁺ cation, while chlorine (Cl) gains one electron to form a Cl⁻ anion. The electrostatic attraction between these oppositely charged ions results in the formation of an ionic bond.
In magnesium oxide, magnesium (Mg) loses two electrons to form a Mg²⁺ cation, while oxygen (O) gains two electrons to form an O²⁻ anion. Again, the electrostatic attraction between these ions forms an ionic bond.
Relate to Everyday Phenomena
Relate the concepts of electron gain to everyday phenomena. For example, consider the process of rusting, which involves the oxidation of iron. Iron atoms lose electrons to form iron ions, which then react with oxygen and water to form rust.
Similarly, consider the process of electrolysis, which involves using an electric current to drive non-spontaneous chemical reactions. Electrolysis can be used to extract metals from their ores, such as aluminum from aluminum oxide. In this process, aluminum ions gain electrons to form aluminum metal.
FAQ: Frequently Asked Questions About Atoms Gaining Electrons
Q: What is an ion? An ion is an atom or molecule that has gained or lost electrons, resulting in an electrical charge. If an atom gains electrons, it becomes a negatively charged ion, called an anion. If it loses electrons, it becomes a positively charged ion, called a cation.
Q: Why do atoms gain electrons? Atoms gain electrons to achieve a more stable electron configuration, typically by filling their outermost electron shell (valence shell). This often involves achieving an octet (eight electrons) in the valence shell, mimicking the electron configuration of noble gases.
Q: Which elements are most likely to gain electrons? Elements with high electronegativity and high electron affinity are most likely to gain electrons. These elements are typically found in Group 16 (chalcogens) and Group 17 (halogens) of the periodic table.
Q: What happens to the charge of an atom when it gains an electron? When an atom gains an electron, its charge becomes more negative. Each electron carries a -1 charge, so gaining one electron results in a -1 charge, gaining two electrons results in a -2 charge, and so on.
Q: How does gaining electrons affect the properties of an atom? Gaining electrons can significantly alter the properties of an atom. Anions often have different chemical reactivity, size, and stability compared to their neutral counterparts. For example, chloride ions (Cl⁻) are much more stable and less reactive than chlorine atoms (Cl).
Conclusion: The Significance of Electron Gain
In summary, when an atom gains an electron, it becomes a negatively charged ion known as an anion. This process is driven by the atom's desire to achieve a stable electron configuration, typically by filling its valence shell. Understanding this concept is fundamental to grasping chemical reactions, ionic bonding, and the properties of matter. From the formation of common salts to the complexities of superhalogens, the gain of electrons plays a crucial role in shaping the world around us.
To deepen your understanding, consider exploring interactive simulations of electron configurations or experimenting with simple chemical reactions that involve ion formation. Are you ready to dive deeper into the fascinating world of chemistry? Share this article, leave a comment with your questions, and let’s continue the exploration together!
Latest Posts
Latest Posts
-
When To Use A Pie Chart
Nov 18, 2025
-
An Inadequate Supply Of Blood To Surrounding Tissues Is Called
Nov 18, 2025
-
Why Do Plants Have Cell Wall And Not Animals
Nov 18, 2025
-
6 Letter Word Starts With An
Nov 18, 2025
-
What Is Negative Multiplied By Negative
Nov 18, 2025
Related Post
Thank you for visiting our website which covers about If An Atom Gains An Electron It Becomes . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.