1s 2 2s 2 2p 6 3s 2 3p 6
catholicpriest
Nov 12, 2025 · 11 min read
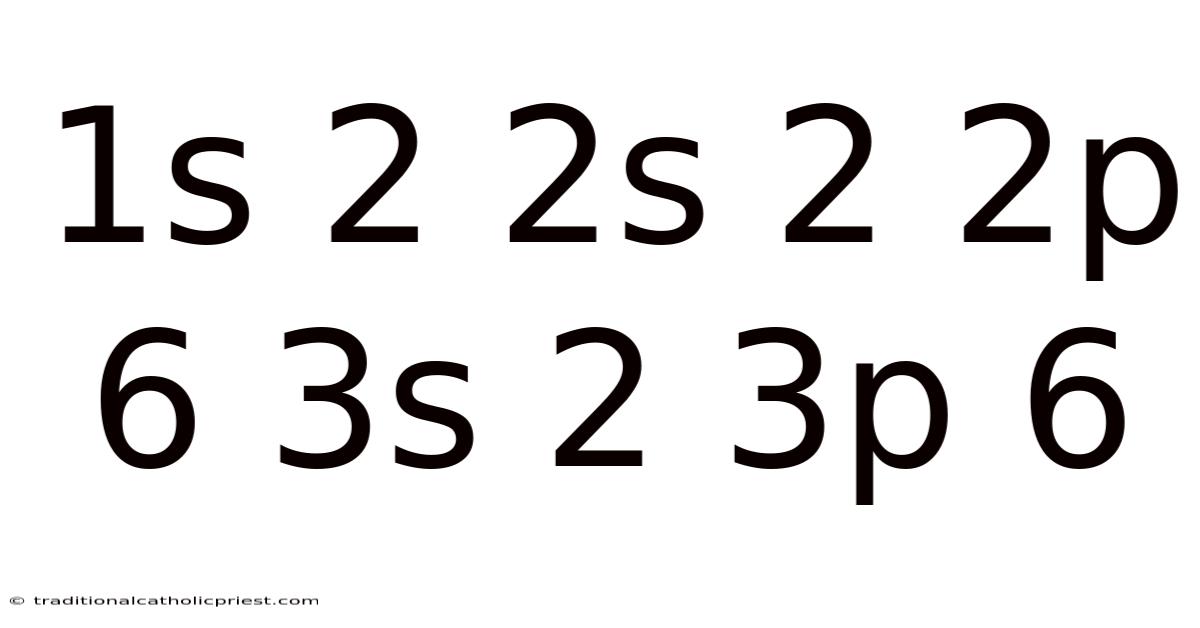
Table of Contents
Imagine you're building with LEGOs. Each brick represents an electron, and you're carefully placing them around a central core. But there are rules: only certain spots are allowed, and each spot can hold only a specific number of LEGOs. This, in essence, is electron configuration – how electrons are arranged within an atom, and understanding the notation "1s² 2s² 2p⁶ 3s² 3p⁶" is like having the blueprint for your LEGO structure. This blueprint tells you exactly how many bricks go where, giving the atom its unique properties.
The electron configuration of an atom dictates its chemical behavior, the way it interacts with other atoms to form molecules, and indeed, the very nature of the substances we encounter daily. Mastering concepts like "1s² 2s² 2p⁶ 3s² 3p⁶" unlocks the door to understanding the complexities of chemistry, from the simplest reactions to the most intricate biochemical processes. In this article, we'll delve deep into the meaning behind this notation, unraveling the underlying principles and exploring its significance in the world of chemistry.
Decoding the Electron Configuration: 1s² 2s² 2p⁶ 3s² 3p⁶
The electron configuration notation, like "1s² 2s² 2p⁶ 3s² 3p⁶," might seem like a cryptic code at first glance, but it's a systematic way of describing the arrangement of electrons within an atom. It reveals which energy levels and sublevels are occupied by electrons, providing crucial information about the atom's properties and reactivity. Understanding this notation is fundamental to comprehending chemical bonding, predicting molecular shapes, and explaining various phenomena in chemistry and materials science.
This notation adheres to a set of established rules and principles governed by quantum mechanics. Each part of the notation represents a specific aspect of the electron's location and energy. For instance, the numbers (1, 2, 3, etc.) indicate the principal energy level or electron shell, while the letters (s, p, d, f) denote the sublevels or orbitals within those shells. The superscripts signify the number of electrons occupying each sublevel. By systematically following these rules, we can decipher the electron configuration of any element and gain valuable insights into its chemical behavior.
Comprehensive Overview: Unpacking the Fundamentals
To fully grasp the meaning of "1s² 2s² 2p⁶ 3s² 3p⁶," we need to break down each component and understand the underlying principles. This involves understanding the concept of electron shells and subshells, the rules that govern electron filling, and the relationship between electron configuration and the periodic table.
Electron Shells and Subshells
Electrons in an atom are organized into distinct energy levels or shells, surrounding the nucleus. These shells are numbered 1, 2, 3, and so on, with higher numbers indicating higher energy levels. The first shell (n=1) is closest to the nucleus and has the lowest energy, while subsequent shells are further away and have progressively higher energies.
Within each shell, electrons reside in subshells, also known as orbitals. These subshells are denoted by the letters s, p, d, and f, each corresponding to a different shape and energy level.
- The s subshell is spherical and can hold a maximum of 2 electrons.
- The p subshell is dumbbell-shaped and consists of three orbitals, each capable of holding 2 electrons, for a total of 6 electrons.
- The d subshell has a more complex shape and consists of five orbitals, holding a maximum of 10 electrons.
- The f subshell is even more complex, with seven orbitals that can accommodate up to 14 electrons.
The notation "1s² 2s² 2p⁶ 3s² 3p⁶" tells us how many electrons occupy each of these shells and subshells.
The Aufbau Principle and Hund's Rule
Determining the electron configuration of an atom involves following specific rules, primarily the Aufbau principle and Hund's rule.
The Aufbau principle states that electrons first fill the lowest energy levels available before occupying higher energy levels. This means that the 1s subshell is filled before the 2s, which is filled before the 2p, and so on. This principle provides the general order of electron filling, but there are some exceptions, particularly for transition metals.
Hund's rule addresses how electrons fill orbitals within a subshell. It states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. Moreover, these single electrons will have the same spin, maximizing the total spin angular momentum. For example, in the 2p subshell, electrons will first occupy each of the three p orbitals individually before any orbital receives a second electron.
Connecting Electron Configuration to the Periodic Table
The periodic table is organized in such a way that elements with similar electron configurations are grouped together. The group number (vertical column) often corresponds to the number of valence electrons, which are the electrons in the outermost shell and are responsible for chemical bonding. The period number (horizontal row) indicates the highest energy level occupied by electrons.
For example, elements in Group 1 (alkali metals) all have one valence electron in their outermost s subshell (e.g., Li: 1s² 2s¹; Na: 1s² 2s² 2p⁶ 3s¹). Elements in Group 17 (halogens) have seven valence electrons (e.g., F: 1s² 2s² 2p⁵; Cl: 1s² 2s² 2p⁶ 3s² 3p⁵). Understanding the relationship between electron configuration and the periodic table allows us to predict the properties and reactivity of elements based on their position in the table.
The Significance of a Filled Outer Shell
The electron configuration "1s² 2s² 2p⁶ 3s² 3p⁶" represents an atom with a completely filled outermost electron shell. In this case, the 3s and 3p subshells are full, giving a total of 8 electrons in the outermost (third) shell. This is a particularly stable configuration, often referred to as an octet.
Atoms with filled outer shells, such as noble gases (Group 18), are exceptionally stable and unreactive. They have little tendency to gain, lose, or share electrons, making them chemically inert. Other elements strive to achieve a similar configuration by gaining, losing, or sharing electrons through chemical bonding, leading to the formation of molecules and compounds.
Ion Formation and Electron Configuration
The electron configuration also helps us understand how ions are formed. When an atom gains or loses electrons, it becomes an ion, carrying either a negative (anion) or positive (cation) charge.
For example, sodium (Na) has an electron configuration of 1s² 2s² 2p⁶ 3s¹. It readily loses its single valence electron to achieve the stable configuration of 1s² 2s² 2p⁶, forming a sodium ion (Na⁺) with a +1 charge. Chlorine (Cl) has an electron configuration of 1s² 2s² 2p⁶ 3s² 3p⁵. It readily gains one electron to achieve the stable configuration of 1s² 2s² 2p⁶ 3s² 3p⁶, forming a chloride ion (Cl⁻) with a -1 charge. These ions can then combine to form an ionic compound, such as sodium chloride (NaCl).
Trends and Latest Developments
In recent years, there has been increasing interest in understanding and manipulating electron configurations for various applications. This includes developing new materials with specific electronic properties, designing catalysts for chemical reactions, and advancing quantum computing technologies.
Computational Chemistry and Electron Configuration
Computational chemistry plays a crucial role in predicting and understanding electron configurations. Sophisticated software and algorithms can calculate the electronic structure of molecules and materials, providing insights into their properties and behavior. These calculations are often based on quantum mechanical principles and require significant computational resources.
Density functional theory (DFT) is one of the most widely used methods in computational chemistry for calculating electron configurations and related properties. DFT allows researchers to model complex systems and predict their behavior without resorting to computationally expensive ab initio methods. These computational tools are invaluable for designing new materials and understanding chemical reactions.
Electron Configuration and Material Science
The electron configuration of elements profoundly impacts the properties of materials. For instance, the electronic structure determines whether a material is a conductor, insulator, or semiconductor. By manipulating the electron configuration through doping, alloying, or applying external fields, we can tailor the properties of materials for specific applications.
For example, in semiconductors, introducing impurities (dopants) with different electron configurations can significantly alter their conductivity. This is the basis of modern electronics, where precise control over electron flow is essential for creating transistors, diodes, and other electronic components.
Advances in Spectroscopic Techniques
Advanced spectroscopic techniques, such as X-ray photoelectron spectroscopy (XPS) and electron energy loss spectroscopy (EELS), provide experimental methods for probing the electron configurations of materials. These techniques allow researchers to directly measure the energy levels and populations of electrons in different shells and subshells, providing valuable information for validating theoretical calculations and understanding material properties.
These spectroscopic methods are particularly useful for studying surface chemistry, catalysis, and nanomaterials, where the electronic structure at the surface or interface plays a critical role in determining their behavior.
The Role of Electron Configuration in Quantum Computing
Quantum computing relies on manipulating the quantum states of electrons to perform computations. The electron configuration of atoms and molecules is crucial in determining their suitability as qubits, the fundamental units of quantum information.
Researchers are exploring various materials and molecules with specific electron configurations that exhibit desirable quantum properties, such as long coherence times and strong interactions with external fields. Controlling and manipulating the electron configuration of these qubits is essential for building practical quantum computers.
Tips and Expert Advice
Understanding and applying the concepts of electron configuration can be challenging, but with the right approach, it can become a powerful tool for understanding chemistry. Here are some practical tips and expert advice to help you master this topic:
Master the Basics
Before tackling complex electron configurations, make sure you have a solid understanding of the fundamentals. This includes:
- Understanding the structure of the atom, including the nucleus and electron shells.
- Knowing the different types of subshells (s, p, d, f) and their shapes.
- Familiarizing yourself with the Aufbau principle and Hund's rule.
- Understanding the relationship between electron configuration and the periodic table.
Without a strong foundation, it will be difficult to grasp more advanced concepts.
Practice, Practice, Practice
The best way to master electron configuration is through practice. Start with simple elements and gradually work your way up to more complex ones. Use the periodic table as a guide and try to predict the electron configuration before looking up the answer.
You can also find online quizzes and exercises that provide instant feedback. The more you practice, the more comfortable you will become with the rules and patterns of electron configuration.
Use Visual Aids
Visual aids, such as diagrams and charts, can be helpful for understanding electron configurations. For example, you can draw orbital diagrams, which show the arrangement of electrons in each subshell using arrows to represent the spin of the electrons.
You can also use color-coded periodic tables that highlight the different blocks (s-block, p-block, d-block, f-block) and their corresponding electron configurations.
Relate to Real-World Examples
Try to relate electron configuration to real-world examples and applications. This will make the concepts more meaningful and easier to remember. For example, you can think about how the electron configuration of different elements affects their properties, such as conductivity, reactivity, and color.
You can also explore how electron configuration is used in various fields, such as medicine, engineering, and environmental science.
Don't Be Afraid to Ask for Help
If you're struggling with electron configuration, don't hesitate to ask for help. Talk to your teacher, professor, or classmates, or seek out online resources and tutorials. There are many people who are willing to help you understand this important topic.
Remember that learning takes time and effort, so be patient with yourself and keep practicing.
FAQ
Q: What is the significance of the numbers in the electron configuration notation?
A: The numbers (1, 2, 3, etc.) represent the principal energy level or electron shell. Higher numbers indicate higher energy levels and greater distance from the nucleus.
Q: What do the letters (s, p, d, f) represent in the electron configuration?
A: The letters denote the subshells or orbitals within each energy level. Each subshell has a distinct shape and energy level.
Q: How many electrons can each subshell hold?
A: The s subshell can hold a maximum of 2 electrons, the p subshell can hold 6 electrons, the d subshell can hold 10 electrons, and the f subshell can hold 14 electrons.
Q: What is the Aufbau principle?
A: The Aufbau principle states that electrons first fill the lowest energy levels available before occupying higher energy levels.
Q: What is Hund's rule?
A: Hund's rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. Moreover, these single electrons will have the same spin.
Q: Why are noble gases unreactive?
A: Noble gases have completely filled outermost electron shells, making them exceptionally stable and unreactive.
Conclusion
Understanding electron configurations, such as "1s² 2s² 2p⁶ 3s² 3p⁶", provides a fundamental understanding of atomic structure and chemical behavior. This notation describes the arrangement of electrons within an atom, revealing insights into its properties, reactivity, and ability to form chemical bonds. By mastering the underlying principles, including electron shells, subshells, the Aufbau principle, and Hund's rule, you can unlock the secrets of the periodic table and predict the behavior of elements and compounds.
To deepen your understanding, we encourage you to explore online resources, practice writing electron configurations for various elements, and consider how these configurations impact real-world applications. Share this article with friends or colleagues who are also interested in chemistry and start a discussion. What other aspects of electron configuration intrigue you? What real-world applications do you find most fascinating? Let us know in the comments below!
Latest Posts
Related Post
Thank you for visiting our website which covers about 1s 2 2s 2 2p 6 3s 2 3p 6 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.